This blog installment focuses on perhaps the most well known example of natural selection in action (and a topic we have covered in the blog before): The peppered moth (Biston betularia). Everyone knows the story, I’m sure, but here’s a brief recap: Peppered moths, a species native to England, naturally occur in two varieties—the light, speckled “typica" and the dark “carbonaria.” During the day, the moths rest on tree trunks, where they are vulnerable to being picked off by hungry birds. Hence, blending in with tree bark provided a strong selection advantage. Before the Industrial Revolution, the vast majority of moths were of the typica variety, but as the coal-powered Industrial Revolution transformed the areas around major cities such as London and Manchester into sooty messes, the 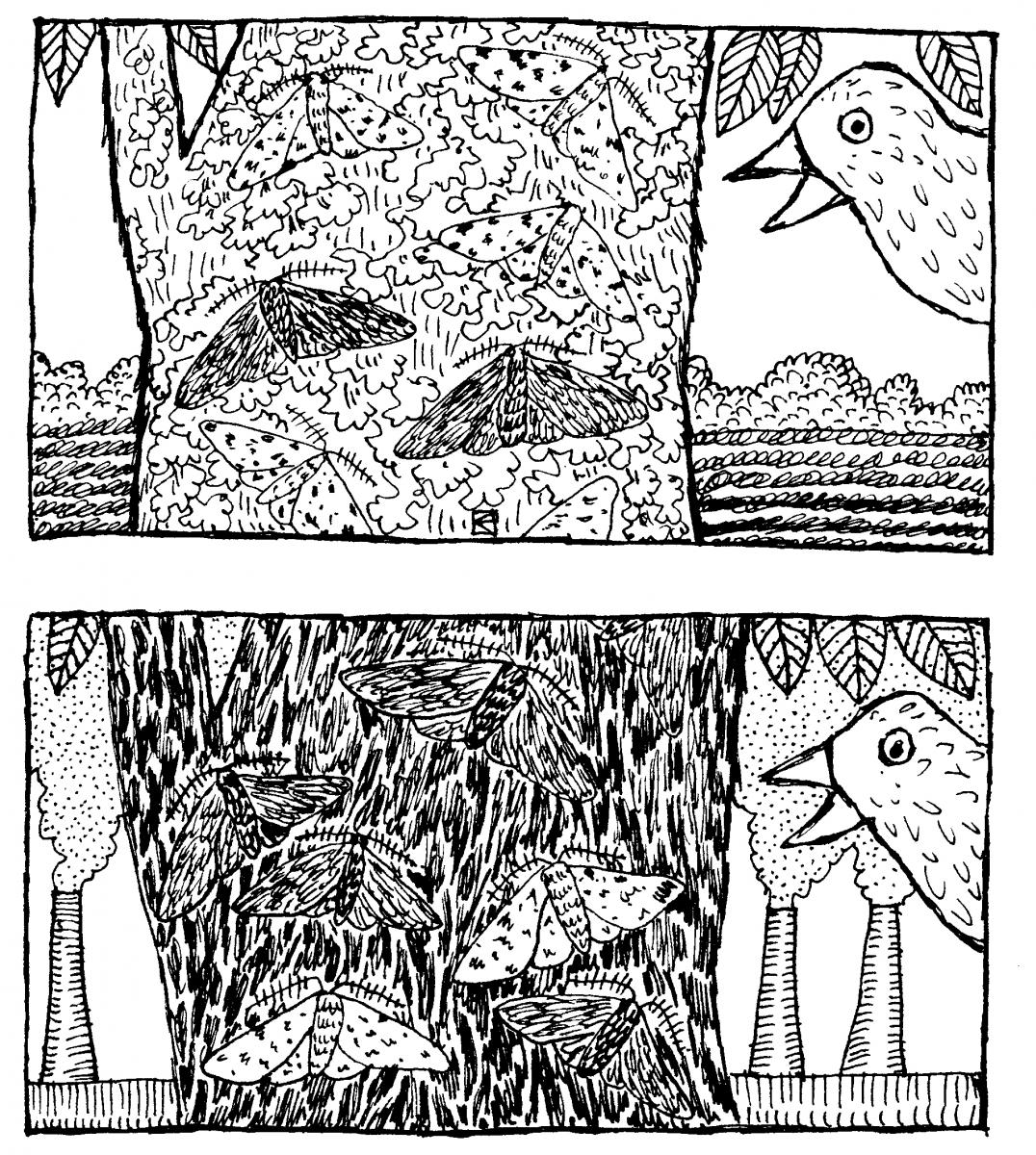 carbonaria variety came to predominate. Then, when the air cleaned up, the distribution of variations shifted back, and the typica moths are once again more common.
carbonaria variety came to predominate. Then, when the air cleaned up, the distribution of variations shifted back, and the typica moths are once again more common.
It’s a lovely, clear example of shifting trait distributions in response to environmental change. Dark coloration made the moths easy prey for birds when tree trunks were in their natural state, but provided greater camouflage when the trunks were coated in black soot. I can’t resist repeating this pun from my original peppered moth post, as it sums things up perfectly and it makes me giggle: You might say that whether a moth survives predation is a matter of whether it’s sooted to its environment.
That the trait shifts were a result of shifting selection pressures (and therefore natural selection) was originally documented in the 1950s by ecologist H. B. D. Kettlewell, and then independently confirmed in the late 1990s by biologist Michael E. N. Majerus. Because this example of natural selection is documented so well and often discussed in textbooks, creationists regularly (and unsuccessfully) try to cast doubt on it. But that’s old news. Why are these moths in the news again in 2016? I’m so glad you asked.
This month, a team of researchers from the University of Liverpool released a study in Nature (frustratingly behind a paywall, but Ed Yong has a nice summary here) announcing that they had found the gene responsible for the peppered moths’ color variation. This was no easy feat—in fact, it took more than five years. The identified bundle of G-A-T-Cs is known as cortex and resides on the moths’ 17th chromosome. You might be thinking at this stage, “Great. But… so?” and I admit I kinda felt the same way. That a single gene controlled the color variation of peppered moths was assumed based on trait distribution (moths were either one color variant or the other with no in-betweens), and frankly it never occurred to me to be curious about this gene at all.
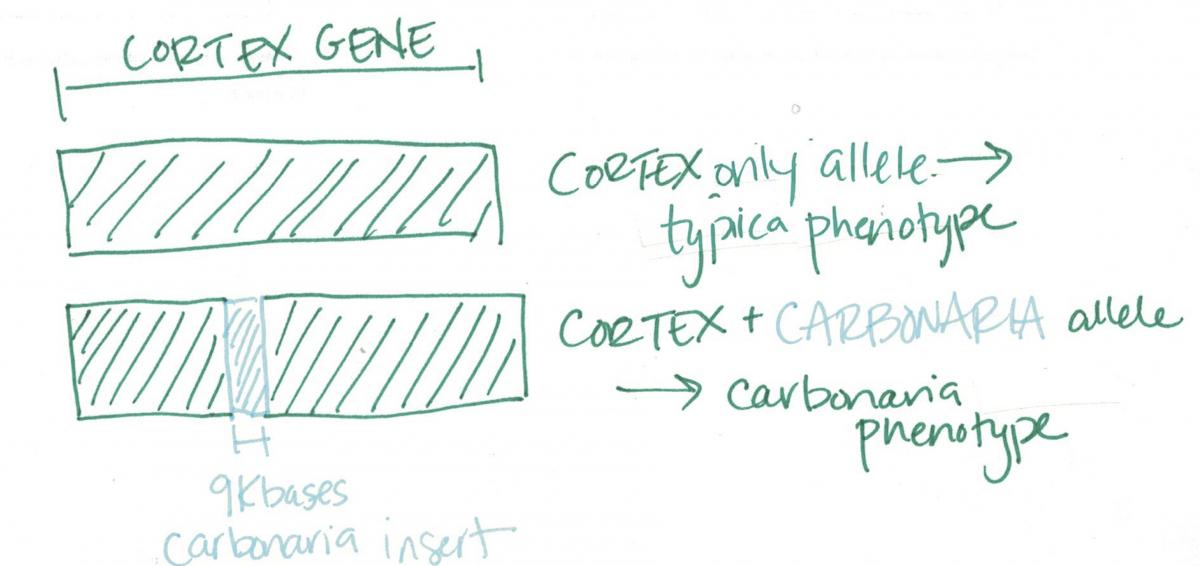 And perhaps, had the cortex gene been a run-of-the-mill gene, it would not have garnered much interest in the press and we wouldn’t be here now. But it turns out that cortex is no ordinary gene—it’s a jumping gene. What is a jumping gene, you ask? It’s a chunk of DNA (also called a transposon) that, well, jumps around the genome. Researchers suspected that a jumping gene was involved in peppered moth coloration when they noticed that the cortex gene of light-colored typica moths lacked a stretch of DNA that was present in their dark-colored counterparts. The missing stretch of DNA is itself a gene, dubbed carbonaria, that is present up to 255 times throughout the moth genome (doing goodness knows what), but it is found nestled itself within the cortex gene, giving the moth dark coloration.
And perhaps, had the cortex gene been a run-of-the-mill gene, it would not have garnered much interest in the press and we wouldn’t be here now. But it turns out that cortex is no ordinary gene—it’s a jumping gene. What is a jumping gene, you ask? It’s a chunk of DNA (also called a transposon) that, well, jumps around the genome. Researchers suspected that a jumping gene was involved in peppered moth coloration when they noticed that the cortex gene of light-colored typica moths lacked a stretch of DNA that was present in their dark-colored counterparts. The missing stretch of DNA is itself a gene, dubbed carbonaria, that is present up to 255 times throughout the moth genome (doing goodness knows what), but it is found nestled itself within the cortex gene, giving the moth dark coloration.
One mystery solved—we know the gene responsible for determining peppered moth color. New mystery opened: how? If you have some basic genetics knowledge, you might think as I did, that the cortex gene with carbonaria insert must code for a different protein than the cortex gene alone does, and this different protein results in dark color when expressed. It’s a good, sensible guess—but it’s wrong. The particular region of the cortex gene that houses the carbonaria insert is an intron—which means the cell discards it before it is translated into a protein. So carbonaria can’t directly effect what protein is built when the cortex gene is expressed because it’s snipped out of the DNA ![]() before the process of expression gets underway! But as it happens, there is more than one way to affect proteins. The researchers suspect that carbonaria somehow affects when and how strongly the cortex protein is expressed. I should mention here that in fruit flies, cortex has a known function controlling mitosis (cell division), so it’s newfound identity as “the peppered moth color gene” was surprising from the outset. But it seems to clearly have a role in pigmentation across a variety of species. Another Nature paper from this month announced the role of cortex in warning coloration among a large diverse group of butterflies (themselves the subject of another famous evolutionary story). These butterflies are not very close cousins to the peppered moths, so it’s probable that cortex plays a role in pigmentation across a wide variety among insects. I feel pretty confident suggesting that these researchers will be busy for a quite some time figuring this all out.
before the process of expression gets underway! But as it happens, there is more than one way to affect proteins. The researchers suspect that carbonaria somehow affects when and how strongly the cortex protein is expressed. I should mention here that in fruit flies, cortex has a known function controlling mitosis (cell division), so it’s newfound identity as “the peppered moth color gene” was surprising from the outset. But it seems to clearly have a role in pigmentation across a variety of species. Another Nature paper from this month announced the role of cortex in warning coloration among a large diverse group of butterflies (themselves the subject of another famous evolutionary story). These butterflies are not very close cousins to the peppered moths, so it’s probable that cortex plays a role in pigmentation across a wide variety among insects. I feel pretty confident suggesting that these researchers will be busy for a quite some time figuring this all out.
One thing that they could already determine, however, is just when the carbonaria transposon jumped its way into the cortex gene. Harnessing the modeling power of computers, the team simulated their way to the conclusion that the “jump” occurred somewhere between 1809 and 1929. Once this mutation was present, it took a while before ecologists first noted it in 1848. This makes sense, given that initially, dark coloration was a liability, so it wouldn’t have spread quickly—but it has persisted, riding the fickle tides of selective pressure and fascinating generations of researchers.
Evolution is so cool.
Are you a teacher and want to tell us about an amazing free resource? Do you have an idea for a Misconception Monday or other type of post? Have a fossil to share? See some good or bad examples of science communication lately? Drop me an email or shoot me a Tweet @keeps3.

