Back in the day, when I was the kind of scientist who worked in a lab, I spent seven years deciphering the genetic sequence of the 1918 influenza virus at the Armed Forces Institute of Pathology (AFIP) in Washington D.C. The pandemic caused by this virus, which erupted in three distinct waves beginning in the late summer of 1918 and ending in the spring of 1919, killed somewhere between 20 and 50 million people worldwide. Never before or since has an influenza virus killed so many, nor returned in so many waves so quickly. Could it happen again? Could the outbreaks of avian influenza (subtype H5N2) currently devastating chicken farms in the Midwest lead to 1918-scale disaster?
I was the kind of scientist who worked in a lab, I spent seven years deciphering the genetic sequence of the 1918 influenza virus at the Armed Forces Institute of Pathology (AFIP) in Washington D.C. The pandemic caused by this virus, which erupted in three distinct waves beginning in the late summer of 1918 and ending in the spring of 1919, killed somewhere between 20 and 50 million people worldwide. Never before or since has an influenza virus killed so many, nor returned in so many waves so quickly. Could it happen again? Could the outbreaks of avian influenza (subtype H5N2) currently devastating chicken farms in the Midwest lead to 1918-scale disaster?
I don’t think so, and let me tell you why.
It was fear that influenza could cause another devastating pan demic that drove Jeff Taubenberger and me, and our colleagues at the AFIP, to attempt to isolate viral genetic material of the virus. Our sources were lung biopsies stored in the AFIP’s repository, and later the frozen lungs of an Inuit woman who died of the flu and was buried in permafrost in Brevig Mission, Alaska in the fall of 1918. At the time, it wasn’t known whether it was even possible to isolate and sequence RNA from such old samples; it had never been done.
demic that drove Jeff Taubenberger and me, and our colleagues at the AFIP, to attempt to isolate viral genetic material of the virus. Our sources were lung biopsies stored in the AFIP’s repository, and later the frozen lungs of an Inuit woman who died of the flu and was buried in permafrost in Brevig Mission, Alaska in the fall of 1918. At the time, it wasn’t known whether it was even possible to isolate and sequence RNA from such old samples; it had never been done.
But the goal—answering questions like, “Where did the virus come from?” and “Why was it was so lethal?”—was so important, we felt we had to try. Long story short: we succeeded. Unfortunately, sequencing the virus’s genes did not provide complete answers to our simple questions about the 1918 pandemic. In fact, the answers remain elusive nearly 10 years later. But that doesn’t mean that we didn’t learn anything. Our work, and that of many other scientists who study influenza, has shed a lot of light on this exceptionally cosmopolitan virus.
I offer this background so that when I tell you that I (and most experts) don’t think this chicken flu (officially called Highly Pathogenic Avian Influenza subtype H5N2, or HPAIH5) will cause a human pandemic, you’ll understand that I’m not completely unqualified to offer such assurances. Viruses, viral evolution, and the factors underlying transmissibilit y and lethality are complicated, but they’re not utterly lawless. And that’s why I get so frustrated when some scientists suggest that because viruses have high mutation rates, they’re basically omnipotent—capable of changing their host range, their mode of transmission and their lethality, seemingly at will. Such claims have been made about not only about bird flu but also about Ebola recently, and I suspect you’ll see this claim emerge (pun intended) in other discussions of newly detected infectious agents. (I wrote about the fears that Ebola would go airborne here).
y and lethality are complicated, but they’re not utterly lawless. And that’s why I get so frustrated when some scientists suggest that because viruses have high mutation rates, they’re basically omnipotent—capable of changing their host range, their mode of transmission and their lethality, seemingly at will. Such claims have been made about not only about bird flu but also about Ebola recently, and I suspect you’ll see this claim emerge (pun intended) in other discussions of newly detected infectious agents. (I wrote about the fears that Ebola would go airborne here).
Now I’m not going to say that viruses can never mutate in ways that enable them to switch hosts, or that result in increased virulence (ability to cause more severe disease). But I will say that it’s far from a given. In fact, it’s pretty unlikely. Viruses—although generally not classified as living things—do share an important characteristic with all living things: they cannot change their characteristics willy-nilly. Viruses cannot evolve on demand, and they’re very specialized, so random mutations are unlikely to be successful, and therefore to spread.
Think about what viruses do—invade cells of another organism and co-opt the cellular machinery to make copies of themselves. This takes considerable precision and specialization. The virus must attach to the cell surface, penetrate th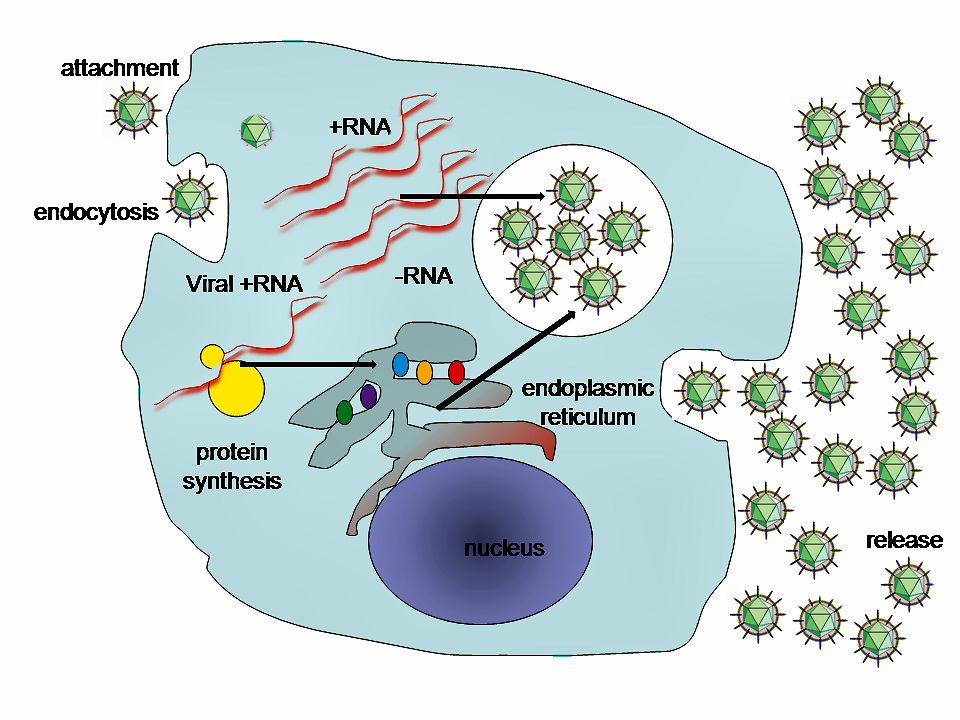 e cell wall or membrane, find its way to a place in the cell where it can unpack its genome, subvert the cell’s own DNA, RNA, and protein-making apparati to make all the various viral components, package the various components up again in the right order and proportions, get all the “baby” viruses to the cell’s surface, and then out of the cell where they can go off in search of more host cells. Phew! And all of this while evading or at least delaying the host’s immune response long enough to accomplish the whole replication cycle.
e cell wall or membrane, find its way to a place in the cell where it can unpack its genome, subvert the cell’s own DNA, RNA, and protein-making apparati to make all the various viral components, package the various components up again in the right order and proportions, get all the “baby” viruses to the cell’s surface, and then out of the cell where they can go off in search of more host cells. Phew! And all of this while evading or at least delaying the host’s immune response long enough to accomplish the whole replication cycle.
It’s sort of amazing that viruses work at all. In my view, the fact that they do what they do, given their tiny size and relatively simple structures, is impressive and fascinating. Like all other biological systems, the interaction between host and virus is both complex and highly specific. A long evolutionary battle has honed each virus’s individual strategy—and this evolutionarily fine-tuned specificity is precisely why chicken-adapted flu strains are highly unlikely to jump to humans, something I’ll talk more about in Part 2.

