INTRODUCTION
It has been more than twelve years since we (Collins 1988, 1997b; Hunt and others 1992) discussed Robert Gentry’s hypothesis proposing that polonium (Po) halos and granite were created nearly instantaneously on Day Three of the Genesis Week (Gen 1:9–10; Gentry 1965, 1970, 1974, 1983, 1988). It is worth examining new information pertinent to the origin of polonium halos. Gentry points out that most granite petrologists believe that all granite bodies of large size are formed deep in the earth’s crust from magma (molten rock) and that as much as 5 million years are required for this magma to be cooled sufficiently for biotite mica to begin to crystallize (see sidebar on p 13 for descriptions of these minerals).
Polonium halos occur in biotite in granites of supposed magmatic origin, and the half-lives of the polonium (Po) isotopes are short (218Po, 3.05 minutes; 214Po, microseconds; and 210Po, 140 days). Gentry claims, therefore, that no matter how much original polonium may have been present in the granite magma, all would have decayed to stable lead (206Pb) in 5 million years, long before the biotite in which polonium halos are found could have formed. He asserts on that basis that polonium halos can be used to support the literal interpretation of the Bible that granite in the earth was created during Day Three of the Genesis Week and not over a period of ~4.6 billion years (Dalrymple 1991). This rapid formation of granite during Day Three and supposed disappearance of polonium isotopes during 5 million years are ideas that are also promoted by Snelling (2008a, 2008b). [Thomas A Baillieul’s detailed summary and critique of Gentry’s views begins on p 17.]
Gentry and Snelling’s claims are without validity (Collins 2008). These creationists ignore the fact that uranium in the original magma would be continuously supplying polonium isotopes during the 5 million years of cooling. The problem is not the disappearance of polonium through 5 million years, as Gentry and Snelling suggest, but the inability of polonium ions produced during this time to migrate from scattered uranium atoms in very viscous magma to precipitate as polonium atoms in a localized place in a growing biotite crystal lattice so that polonium halos can form. The question to ask, therefore, is: how has it been possible for uranium to concentrate in local sources so that polonium, which is derived from the decay of this uranium, could nucleate in growing crystals of biotite or fluorite? There are two possible mechanisms to make this concentration happen. The first is by the formation of either vein-dikes or pegmatites containing uranium minerals that are associated with chemical replacement processes (metasomatism). The second is by the formation of pegmatites containing uranium minerals that result from magmatic processes. Both mechanisms are examined in this article.

|
| FIGURE 1. Myrmekite (center; white vermicules [quartz]; black [plagioclase feldspar]) adjacent to potassium feldspar with grid twinning (bottom; black and white) with cross-polarized light. |
POLONIUM HALOS IN VEIN-DIKES AND PEGMATITES ASSOCIATED WITH CHEMICAL REPLACEMENT PROCESSES
Collins found that in many places where poloniumhalo- bearing biotite and fluorite crystals are present, the adjacent granitic rocks were microfractured and contained myrmekite (the intergrowth of plagioclase and vermicular quartz) (Collins 1988, 1997a; Figure 1). The granitic rocks in these places were produced by chemical replacement processes (metasomatism) of previously solidified igneous rocks at temperatures below those required for melting (350–550ºC).
The evidence that such granite is formed by metasomatism consists of detailed thin section studies, electron microprobe analyses, and cathodoluminescence images of undeformed diorite through a transition of deformation into granite where calcium, sodium, iron, magnesium, and aluminum were subtracted as potassium and silica were introduced (Collins 2002). The resulting metasomatic granite that is formed at temperatures below melting conditions looks like granite that has crystallized from magma because the newly formed granite inherits the mineral textures and structures (dikes into wall rocks and inclusions of foreign rocks) of the original igneous rock (for example, diorite), but now the rock’s mineral and chemical compositions have been changed into what occur in granite. The process is similar to the formation of petrified wood in which silica atoms are brought in and exchanged for carbon atoms, preserving the cellular structure of the wood, except that in the metasomatic granite, potassium is exchanged for sodium and calcium, converting plagioclase feldspar into potassium feldspar while preserving the original shapes of the plagioclase crystals. Some of these same myrmekite-bearing microfractured granitic rocks contained scattered but relatively abundant uranium (238U) in crystals of uraninite and zircon, so a nearby source for radioactive radon gas (222Rn) was readily available, as were polonium 218Po, 214Po, and 210Po, the three daughter isotopes of 222Rn. Fracturing of the rock that is intense enough produces open spaces that became filled with the dissolved elements that ultimately formed calcite veindikes containing biotite (and fluorite) with polonium halos (Wakefield 1987–8, 1988). Coarse-crystalline pegmatites were produced in other places in this same area when closely spaced microfractured rocks were converted to granite by replacement processes. Uranium continued to supply large amounts of radioactive radon and polonium once it became concentrated in, or was in route to, these lower-pressure microfractured places. This accumulation of radioactive elements in the lower pressure sites enabled polonium to nucleate in growing or recrystallizing crystals of biotite mica (and fluorite).
Polonium ions nucleate in biotite and fluorite because these ions are large and can fit only in large sized holes in a mineral lattice. Such holes occur in biotite and fluorite but not in the other kinds of minerals commonly found in granite. The polonium ions nucleating on the faces of growing biotite crystals and fluorite subsequently became enclosed inside these crystals. The enclosed polonium ions would then begin to decay and emit alpha particles. The alpha particles, shot out in random patterns, would cause damage to the crystal lattice producing spheres with different radii, destroying the lattice structure and producing a disordered pattern, known as a glass, which appears as a black circular spot under the petrographic microscope. Rings of these different radii of damage can be seen if these spheres are cut through in the plane of the equator. Such rings are referred to as halos — hence, 218Po, 214Po, and 210Po halos. From 9 to 10 billion atoms of polonium are needed at a nucleation point before individual halos can be seen (Gentry 1988). This means that vast numbers of polonium atoms were once present in the crystals of biotite before these atoms all eventually decayed to stable lead (206Pb).
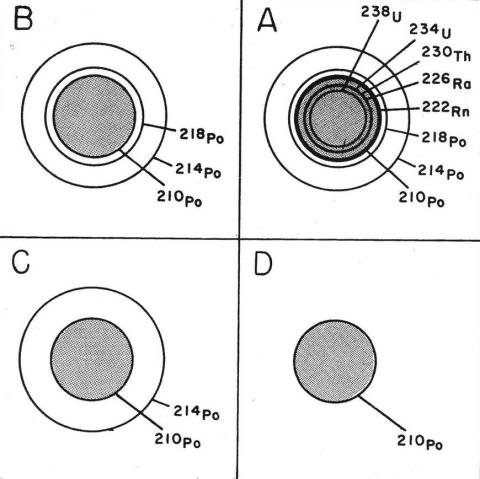
Examples of the three types of polonium halos can be seen below the geologic map of Wakefield (1987–8). Figure 2 shows schematic diagrams of the rings (halos) of damage for the three polonium halo types and for a uranium halo. The uranium-halo schematic shows that the three polonium isotopes are the last three daughter isotopes in the eight-step decay of 238U, each step losing a mass of 4. On that basis, the 218Po halo with its three rings, the 214Po halo with its two rings, and the 210Po halo with its one ring are isolated (separate) from any immediate uranium source, but, of course, the polonium ions that nucleate to produce these halos are derived from some nearby uranium source.
Numerous polonium halos occur per cubic centimeter in the biotite “books” in the calcite vein-dike of the Silver Crater Mine (figure 14 of Wakefield 1987–8). (Numerous could mean 20–30 thousand polonium halos per cubic centimeter in biotite as reported by Gentry [1968] in a Norwegian mica.) Biotite at the Silver Crater Mine and fluorite in calcite vein-dikes in the Wilberforce area show no evidence of any fracturing that would provide avenues along which radon gas and ions of polonium could move to nucleation points. The growing crystals of biotite and fluorite would be inside large volumes of hot gaseous fluids occupying the open fracture and would not be expected to be fractured or deformed. The Wilberforce area can be seen on the edge of the geologic map in Wakefield (1987–8) west of the Fission Mine where the last four letters “orce” appears.
After crystallization in the calcite vein-dikes, ongoing replacements can continue to occur, involving multiple deformations in the adjacent granitic rocks that allow more fluids to come into the vein-dikes. Both biotite and apatite are reported to replace calcite in some of the vein-dikes in the Bancroft area (Wakefield 1987–8, 1988). These second growth biotite crystals also contain polonium halos, indicating that the hydrous fluids causing these additional replacements carried 222Rn and polonium isotopes.
Because the location where Gentry (1988) reported some of his best polonium halos in biotite (and fluorite) was not in granite but in a calcite vein-dike near the Silver Crater uranium mine in the Bancroft area of Ontario, Canada (Gentry 1971, 1974; Wakefield 1987–8, 1988), his claim for nearly instant crystallization of granite is immediately nullified. In his model, all biotite containing polonium halos crystallized in granite formed from magma. Calcite vein-dikes, however, do not form from granite magma at any stage of its crystallization. Such vein-dikes fill fractures (a meter or more wide) in previously solidified granitic rock and are, therefore, later than the crystallization of the granite (Wakefield 1987–8, 1988).
FRACTURES IN BIOTITE WITH POLONIUM HALOS IN PEGMATITES
Gentry claimed that biotite (and fluorite) crystals containing polonium halos always lacked any microfractures through which radioactive radon or polonium ions could penetrate to produce the polonium halos. This is not true. The adjacent granitic rocks have microfractures where pegmatites were formed by recrystallization and replacement, such as the Buckhorn pegmatite in the Bancroft area. The crystals of biotite crystals in such places that contain polonium halos show evidence of microfracturing or evidence that they had microfracturing prior to recrystallization (Collins 1997b).
For example, in Figure 3, a band of alpha-particle damage can be seen where radioactive inert 222Rn gas and 210Po and 218Po ions were once migrating in fluids along a fracture in the crystal of biotite. These three isotopes produce radii of damage that are almost the same distance from the center line of the fracture so they are not distinguishable from each other in the band of continuous overlapping damage which produces a smeared-out band of damage to the lattice of the biotite. Billions of radioactive isotopes once moved along this fracture, shooting out alpha particles as the fluids progressed through the fracture. Sufficient 210Po (9 billion atoms or more) nucleated at one place along the fracture to create the isolated black 210Po spherical halo of damage. Another example of a fracture containing a 210Po halo in biotite is shown in figure 4 of Collins (1997b). The assertion by Gentry that polonium halos are never found along fractures in biotite is not true.
RATE AT WHICH BIOTITE CRYSTALS OF LARGE SIZE GROW
Gentry believes that granite and polonium-halo-bearing biotite had to form nearly instantly on Day Three of the Genesis Week. He argued, therefore, that the rate of crystallization of granite must be exceedingly fast. Snelling (2008b) suggested that the granite formed in 6–10 days. In contrast, silicate crystals (quartz, mica, feldspars, and so on) in deep-seated magma normally grow exceedingly slowly (over thousands and millions of years) because (1) the heat in molten rock at great depth escapes only very slowly to the earth’s surface so that the rate of cooling is very slow, (2) the high viscosity of the silica-rich melt (like a hot, thick, molten, silica glass) prevents metallic ions from diffusing quickly to nucleation and growingcrystal- sites, and (3) water (steam) that would facilitate rapid diffusion of such ions is generally absent.
However, the rate at which silicate crystals grow in granite pegmatites (where large crystals several centimeters wide may form) can be rapid because of the local great abundance of water (steam). The abundant water occurs because water tends to concentrate in localized volumes in late stages of crystallization of magma because most minerals crystallizing in granite lack any water in their lattices, and it is where abundant water is present that pegmatites form. Crystals in pegmatites can grow to large sizes in a matter of a few days or weeks (London 2008; Nabelek and others 2009; Sirbescu and others 2008; Webber and others 1999).
The rate of growth of calcite and biotite in fluids where calcite vein-dikes form must be even faster than the rate of growth for silicate minerals crystallizing in pegmatites in a granite body. The fluids that produce the calcite vein-dikes would have a high water content and notably low silica so they would have low viscosity. The growth of large crystals of biotite (and fluorite) crystals could, perhaps, be in a matter of hours or less, and, therefore, the growth of superposed lattice layers would also surround nucleating polonium ions on the faces of the growing crystals. Thus, thousands of polonium halos per cubic centimeter in crystals of biotite and fluorite are possible lacking any evidence for microfractures.
FORMATION OF GRANITE BY CHEMICAL REPLACEMENT PROCESSES CONFIRMED
Gentry rejected the model for granite’s being formed by chemical replacement processes because there was no publication in refereed geology journals of a large-scale chemical-replacement model for the origin of some granitic masses. However, several recent studies have indicated the presence of large scale metasomatic (replacement) processes. Andrew Putnis and colleagues, using microprobe studies on an atomic scale, have confirmed that a chemical replacement model for the origin of some granite masses is correct (Putnis and others 2007; Engvik and others 2008; Plumper and Putnis 2009). The evidence for this replacement is the presence of numerous tiny pores in plagioclase feldspar crystals in primary igneous rocks that were deformed and microfractured. Fluids moved through these pores and brought in potassium and/or sodium while depositing tiny rosettes of red hematite crystals along the walls of the pores. The introduction of the potassium and/or sodium converted large masses of igneous rocks into granitic rocks (with surface areas several kilometers in diameter) in Finland, Sweden, Brazil, and California. Where potassium was introduced, myrmekite (similar to that in Figure 1) locally borders the potassium feldspar in these rocks (Collins and Collins 2002). The presence of myrmekite alone can indicate that the rock system was open to ready movement of fluids that could have contained dissolved radioactive radon and polonium ions (if available). Myrmekite is formed locally where chemically altered lattices of relatively calcic plagioclase are incompletely replaced by potassium feldspar, leaving residual calcium, sodium, aluminum, and silica atoms in the lattice which are not in proper balance to recrystallize only as more sodic plagioclase; so some silica is left over to recrystallize as quartz vermicules (Collins 1997a). During metasomatism the reactions do not occur in balanced mass-for-mass exchanges, as one is taught in chemistry classes, but by volume-for-volume exchanges of elements (ions) in minerals that have different densities.
Other published examples of large-scale chemical replacements by potassium feldspar come from economic geologists. For example, Doucette (2000) reports that such replacements occur in volcanic porphyry where gold and copper enrichments are found, converting plagioclase phenocrysts into potassium feldspar (see figure 27 in Doucette 2000). Large-scale potassiumfeldspar replacements, extending over hundreds of square kilometers, are reported by Liu and others (2003) in the uppermost Precambrian rocks underlying Paleozoic sedimentary rocks in the North American mid-continent.
POLONIUM HALOS FORMED BY MAGMATIC PROCESSES
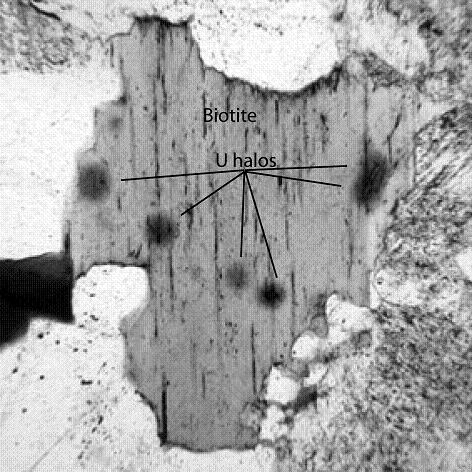
Uranium halos are commonly found throughout a granite mass; isolated polonium halos are rare or absent (Figure 2). Uranium in magma is incorporated into crystals of zircon or uraninite as the magma cools and solidifies. The element preferentially enters into zircon’s crystal structure because uranium’s ionic charge (4+) is the same as that of the zirconium ion. Cooling magma is normally too viscous for large amounts of uranium or zirconium ions to diffuse. Most uranium and zirconium ions, therefore, move only very short distances, precipitating in tiny crystals of uraninite or zircon, or the uranium is precipitated only in crystals of zircon, which are scattered throughout the granite mass. These tiny zircon or uraninite crystals are then enclosed in, or fill spaces in between, other silicate minerals that are crystallizing in the granite mass, such as quartz, feldspars, and biotite. Once the crystals in the granitic mass have formed, any polonium that would be produced must be derived from the decaying uranium in the zircon crystals that had already nucleated and been incorporated in the crystallized biotite and could not then nucleate in later-formed biotite to create visible isolated polonium halos. Only 238U halos including eight spheres of damage surrounding the zircon (or uraninite) crystals would be produced following the solidification of granite magma. These eight spheres have a common center where the uranium is concentrated, and each sphere has a different radius that corresponds to the energies of emissions of alpha particles from each of the eight daughter isotopes in the 238U decay series until stable lead 206Pb is formed (Collins 1997b). The last three isotopes in the 238U decay series are 218Po, 214Po, and 210Po, so their halos are part of the eight produced in the adjacent biotite bordering the uranium source and do not occur as separate isolated halos (Figure 2). The damage in biotite surrounding 238U-bearing zircon crystals in granite can be seen as circular or oval black halos outlining the shapes of the zircon (or uraninite) crystals under the petrographic microscope (Figure 4). The thin section is too thick, however, to see the eight separate halos.
It is also important to point out that the ratio of the amount of lead 206Pb to the amount of the remaining uranium 238U in zircon crystals is used by geochronologists to determine the age of the crystallization of the granite. Because the age determined by this method is consistent from place to place in the same granite (within experimental error), this consistency indicates that the decay process for 238U obeys natural laws that are not arbitrary, which in turn validates the use of this method for determining the age of a granite body (Dalrymple 1991). This applicability in two (or more) places of the results of a single principle based on the observation of natural processes in a way that is consistent with findings from other models and methods of analysis, such as rubidium-strontium (Rb-Sr) and potassium-argon (K-Ar) age determinations, reinforces the validity of the uranium-lead (UPb) age determinations.
The formation of isolated polonium halos in magmatic pegmatites, on the other hand, is possible because of the very large atomic size of the uranium atom (ion), which causes some uranium atoms to be concentrated in both zircon crystals and in residual hydrous fluids during last stages of crystallization of granite magma. Atoms (ions) that are either too small or too large to fit in stable arrangements in holes in the lattices of such silicate minerals as biotite, plagioclase feldspar, and potassium feldspar that are common in magmatic granite are left over in the residual fluids of the last stages of solidification of granite magma (Klein and Hurlbut 1985). For example, smallsized atoms (ions) of lithium, beryllium, and boron (elements numbers 3, 4, and 5) are commonly crystallized in late-forming pegmatites in gem minerals. Atoms that are too large include gold (element number 79) and uranium (element number 92). Uranium is commonly found in scattered zircon crystals in granite (as noted above), but some uranium may also be concentrated in late stages of granite crystallization in pegmatites in the mineral uraninite because of its very large atomic size. Biotite and fluorite crystallizing near this uraninite could plausibly contain Pohalos because the concentrated uranium atoms in this uraninite and in fluids bringing this uranium to the pegmatites would be an abundant source of radon 222Rn and polonium isotopes.
Gentry (1988) actually includes an illustration of a large biotite crystal containing polonium halos in a pegmatite from Murray Bay, Canada. This pegmatite contains crystals of beryl, zircon, and uraninite (Spense 1940). The association of the gem mineral beryl with biotite probably indicates an origin by crystallization of this pegmatite from magma. The biotite would not be microfractured in such an environment, and the presence of abundant steam would permit rapid growth of large crystals. This mineral association in no way indicates that the pegmatite had to crystallize nearly instantaneously. Furthermore, biotite crystals in pegmatites that lack uraninite also lack polonium halos. The presence or absence of polonium halos in biotite in magmatic pegmatite is directly related to the presence or absence of nearby uranium in uraninite or zircon and not because of instant cooling.
CONCLUSIONS
The absence of microfractures in some polonium halo-bearing biotite and fluorite is plausibly explained where these minerals grew in former large, open fractures that were ultimately filled mostly by calcite. In microfractured granitic rocks that were modified by metasomatic processes, polonium halos can form along microfractures in biotite. The rapid rates at which crystals can grow in calcite vein-dikes or pegmatites in the presence of steam and the rapid rates at which radioactive isotopes can diffuse from areas of relatively high pressures into possible large open fractures are important factors in the formation of polonium halos. The coexistence of uranium-bearing minerals in calcite vein-dikes or pegmatites which release abundant amounts of radioactive radon 222Rn and the easy transport or diffusion of uranium and polonium ions and neutral radon gas in surrounding microfractured rock are also necessary. Also important is the relatively long half-life of 222Rn (3.82 days). All these factors provide the means by which polonium halos are formed in biotite and fluorite by natural processes.
The absence of microfractures in biotite (or fluorite) where these minerals grew in pegmatites that were crystallized during the last stages of the solidification of granite magma is plausibly explained because crystals forming in magma are generally not microfractured. Moreover, it is plausible that polonium halos can form in pegmatites of magmatic origin because of the transport or diffusion of uranium and polonium ions and neutral radon gas in steam that is concentrating in local places during the last stages of crystallization of granite magma.
Granite bodies of both primary magmatic and secondary chemical replacement origins are not created during a single young age in the Genesis Week but are among a continuum of ages that range from early in the Precambrian to the late Cenozoic. The one essential requirement for polonium halos to form in biotite and fluorite in calcite vein-dikes or granite (either produced by chemical replacement processes or by magmatic processes) is the nearby presence of uraniumbearing minerals that supply the large quantities of radon 222Rn and polonium.
If polonium halos truly had a nearly instantaneous origin as suggested by Gentry (1988), then even more examples of other polonium halo types would be expected to occur, such as (1) halos of 215Po and 211Po that are derived from radon gas 219Rn in the radioactive uranium (235U) decay series or (2) halos of 216Po and 212Po that are derived from radon gas 220Rn in the radioactive thorium (232Th) decay series. But they are not found (Collins 1997b). The reason is that the radon gas atoms (219Rn and 220Rn) in these two decay series which are the precursors for the other radioactive polonium isotopes have half-lives in seconds, and their daughter polonium isotopes have half-lives in seconds and microseconds instead of 3.05 minutes for 218Po and 140 days for 210Po in the 238U decay series (Collins 1997b). However, Gentry found only one kind of Po-halo sequences among three possible kinds in biotite and fluorite of supposed instantaneous origin.
ACKNOWLEDGMENTS
We wish to thank Mona Sirbescu for providing information regarding rates of crystallization of pegmatites. We express our appreciation to Richard Wakefield for correcting information about the geology and descriptions of the vein-dikes in the Bancroft area of Canada, Steve Lipshie for editorial suggestions and checking the clarity of the article, Forrest Hopson for suggesting many editorial changes and for annotating illustrations, and John Doucette for recommended style changes in writing the article.