| The following correction was subsequently made to this article in issue 12 (volume 4.2): |
| There was an omission in Andrew J. Petto's article, "The Turtle: Evolutionary Dilemma or Creationist Shell Game?" in Creation/Evolution X. Figure 2 was derived from a similar figure published in Vertebrate Dissection by W. F. Walker, 1970 (Philadelphia: W. B. Saunders). Therefore, the caption should have credited this source. |
For the Wyandot Indians of the central plains of North America, the world grew from a few grains of earth from the bottom of the sea spit onto the back of Big Turtle by Old Toad. The world of the Onandaga was formed by Muskrat who placed earth on Snapping Turtle's back. In several Hindu myths, the god Vishnu, in his second incarnation as a turtle, retrieves earth from the bottom of the sea. On his back stands the elephant whose shoulders support the earth (Reeve, 1975).
In these cultures, the world rests on the shell of a turtle. In our culture, the turtle bears the weight of an anti-evolutionary argument on its back as well. How, ask "scientific creationists," could the turtle evolve ribs on the outside of its shoulders from ancestors who are built the other way around? And why do no other descendants of those reptilian ancestors share this arrangement?
Bolton Davidheiser (1971) wonders:
If the turtle evolved from animals of a more "orthodox" structure, it is a mystery how they managed to get their shoulder bones inside their rib cages. If they were outside the ribs, as in other animals, they would also be outside the shell. (p. 246)
In other words, Davidheiser believes that the turtle's shoulder and rib arrangement is too different from that of other animals and too complex to have arisen through evolutionary processes.
Klotz (1979) voices a second objection to the evolution of the turtle. He claims that there is no evidence, in the form of intermediate or transitional forms, of shared ancestry between turtles and other reptiles:
All reptiles are supposed to have developed from the stem reptiles, the cotylosaurs. A modified cotylosaur, Eunotosaurus, is sometimes postulated as the ancestor of all the turtles in that it comes from the proper time and it appears to be on the verge of developing a shell. But it has one serious drawback as a turtle ancestor.
The carapace of modern turtles does not develop just from wide ribs but from independent plates of dermal bone which expand markedly and fuse with one another and with the underlying ribs and any shell or plastron. This unique armor and the contortions which the skeleton had to undergo to fit into it, combined with the toothless beak, have suggested to some that turtles are entirely different from any living reptile. (p. 457)
Creationists argue that the first turtle found in the fossil record is clearly a complete turtle, not some intermediate or transitional form on the way to becoming a turtle from a more conventional reptile.
Davidheiser's denial of a plausible evolutionary pathway to account for the transition attempts to deal a fatal blow to the evolution of the turtle.
It was hoped that a study of the embryonic development of the turtles would clarify this. The problem is discussed by Archie Carr, professor of biological sciences at the University of Florida. He says, "It might accordingly have been hoped that the evolution of the relationship between the shell, ribs, and the girdles during embryology would shed some light on the original history of these events, but such is not the case. (p. 246)
This passage suggests that Carr finds no evidence of a reasonable link between the development of the turtle embryo and evolutionary changes in the turtle lineage.
The creationist challenge appears formidable since it attacks the roots of evolutionary biology. The main arguments dispute similarity of form, shared ancestry, and a plausible evolutionary pathway from the stem reptiles to turtles. Yet as formidable as this claim appears, there is little substance in the creationist objections. A careful examination of these arguments against turtle evolution will show why.
The key question is: Do turtles start with the same basic structures as other reptiles and change during their growth and development or are they truly different from the moment they begin to take shape? The answer will determine the strength of the other creationist objections.
Turtle Embryology
Studies of a developing embryo are useful in evolutionary biology. Stephen Jay Gould shows how small changes in the rate and timing of developmental processes can result in major changes in the form of the adult animal (1977:257-260). An unusual feature in an adult animal might be the result of such a developmental change or might be a new feature unique to a particular group of animals.
Does Carr really fail to find any reasonable link between the turtles and other reptiles, as Davidheiser claims, or is Carr a victim of the creationist tactic of selective citation? Davidheiser's citation was taken from the introduction to Carr's Handbook of the Turtles, which reads in part:
In many cases, some inkling of the historical origin of an anatomical feature may be gained by studying its development in a growing embryo. The occurrence of lateral folds in turtle embryos, for instance, probably means that the ancestral form had these structures, which today are found only in lizards. It might accordingly have been hoped that the evolution of the relationship between the shell, ribs, and the girdles during embryology would shed some light on the original history of these events, but such is not the case. (p. 3)
Carr goes on to discuss research which has shown that the turtle conforms to expectations for an animal maintaining a conservative reptilian embryology with an anatomical specialization for external armor. In this context, Carr's comments argue for the reptilian ancestry of the turtle, not against it as Davidheiser would have us believe.
Carr says that the embryology of the turtle confirms that it is a "good" reptile. No new or unusual—nonreptilian—structures appear in its embryology. His disappointment that his study failed to reveal much about the historical origin of the turtle's specializations was based on his observation that turtle development follows rather ordinary pathways. If the process of turtle growth and development is so ordinary, then what explains the unusual result?
Growth and Development of the Shoulder Girdle and Shell
Walker (1947) follows the development of the turtle shoulder girdles from the stage at which limb tissues are first recognizable (when the embryo is 9.5 mm in length) to the stage at which the formation of elements is complete, except for the growth to hatchling size (32 mm). Two significant events occur in the turtle development (ontogeny) which account for the relationship between ribs and the shoulder girdle.
First, the ribs become associated with the shell covering the back (carapace). They do not extend belly-ward to enclose the body. Since the ribs do not surround the body to attach to the middle of the chest, there is no breastbone.
Second, the shoulder girdle becomes associated with the bottom shell (plastron). Parts of the shoulder girdle which are formed from dermal bone actually become incorporated into the plastron. Both these developments confirm the importance of the specialization for external armor in the development of the rib-shoulder relationship.
When the carapace is formed, its embryonic model (anlage) begins as a narrow band of tissue running down the middle of the animal's back. The rib models are short and very strongly associated with the carapace even at this early stage (embryo length: 9.6 mm). Over the next few stages, the shell development dominates body growth, and the whole embryo becomes wide and shallow. The narrow band expands, and the developing carapace carries the ribs with it.
At this stage the "collarbones," also a part of the shoulder girdle, are strongly associated with the developing plastron. Soon (when the embryo reaches 11 mm in length) the collar bones are completely contained within the plastron, and the bony shields of both shells begin to harden. In short, the shoulder girdle does not change its position. The ribs become fused to the shell and are carried outward during the course of development.
The ribs and the limb bones are formed as models in cartilage before being replaced by calcified tissue which later becomes bone. This type of bone is called replacement bone, since cartilage models are replaced by bony tissue.
The bony shields of the turtle shell form directly in the skin without being formed first in cartilage. It belongs in the category of dermal or membrane bone, and it is prominent in fishes and early land animals. The collar bones are dermal bone and are incorporated into the plastron during development. This close association between developing plastral bone and conservative reptile dermal elements in the abdomen and chest skeleton is common among reptiles (Zangrel, 1969). In turtles with reduced shells, the plastra are not platelike. They are rodlike and are distributed in a pattern similar to the pattern of dermal bone in the abdomen and chest of ancient reptiles.
The development of the turtle shell accounts for most of the unique features of these animals. The basis of the turtle adaptive shift is the development of the body armor. The primacy of the shell in development confirms its importance as a basic feature of turtle adaptation. Yet, except, for the incorporation of certain bones into the shell, other aspects of turtle development follow the general reptilian p, attern, as Carr states.
The embryonic development of the turtle tells us many things. First, the "unusual" features arise from a very ordinary reptilian embryo. Shared ancestry with other reptiles is confirmed. The structural similarities between turtles and other reptiles are great indeed.
Second, the developmental changes involved are simple. The timing and intensity of the outward growth of the embryonic structures are altered. This occurs at a time before muscle attachments have formed, avoiding problems f, or limb function at a later stage. The rib cage alters its position, but the shoulder girdle develops as usual for a reptile. Similarity of form is maintained. A plausible evolutionary pathway exists: enclose a reptile in a shell of dermal bone and horny scales.
The Form of the Shoulder Girdle
In land animals that walk on all fours, the shoulder girdle consists of a combination of bone and muscle which provides the strength for movement and support. In the earliest land animals, the bones of the shoulder girdle formed a robust
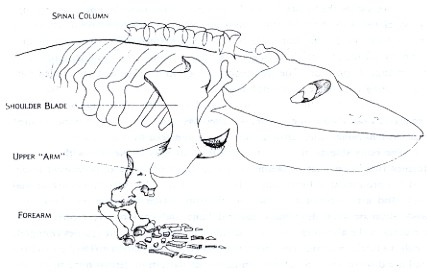
Figure 1: Eryops megacephalus, an early land animal that has typically short ribs which do not enclose the body. The shoulder girdle is a basin of heavy bone set very close to the animal's head. Without a great deal of competition on the land, the biggest problem for Eryops to solve is that of a stable support for its large body and heavy head.
basin on which the body rested (see Figure 1). In modern mammals, much of the support is provided by a muscular sling from which the body is suspended (see Figure 2).
The differences between these two ways of supporting the body are similar to the differences between a hammock and an army cot. A hammock resembles the muscular sling used by land mammals. The supporting columns are spaced off to the sides, and the weight they support is suspended on a flexible material stretched between them. This makes the whole structure light and easy to move.
The army cot approach is more common among reptiles-especially the earliest land animals. The support columns are connected to a sturdy frame which is directly underneath the weight they support. The same sort of flexible material is used, but it is stretched taut over the frame. The main task of support falls to the frame itself. This makes the whole structure very strong and stable but heavy and more difficult to move.
These differences may be seen in Figures 2 and 3, which are drawn from models of cat and turtle shoulder girdles, respectively. In the cat, mobility is favored over stability in the shoulder girdle. The animal is able to move, change directions, and adjust quickly to changes in terrain. In the turtle, stability and strength are emphasized. The protection offered by a strong armor is the key to its success. The bones of its shoulder girdle can be compared with those of the ancient land animal in Figure 1.
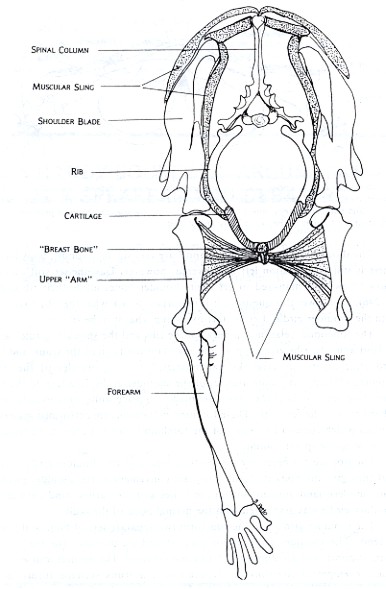
Figure 2: Front view of Felis domestica, the house cat, shows the general relationship between the shoulder girdle and forelimb for running mammals. The ribs extend most of the way to the belly and are connected by cartilage to the breast bone. The shoulder blades are connected to this bone on each side by a combination of relatively reduced collar bones and a sling of muscles and connective tissue. This arrangement makes the whole complex light and maneuverable. This is important for an animal which hunts active prey and performs a lot of different locomotive activities such as running, jumping, and climbing.
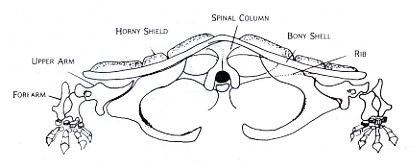
Figure 3: Front view based upon the skeleton of Chelydra, the snapping turtle shows the basinlike shoulder girdle typical among primitive land animals. The space between the two girdles in the bottom shell is where the two join with the bones of the lower shell. Since the shell provides protection and hunting strategy differs from that of the running animals, the main problem is again that of strong support of the heavy body and stability.
The shoulder girdles in Figures 1 and 3 are very similar—robust, bony basins. There is still one question left unanswered, however. Davidheiser still wants to know "how they managed to get their shoulder bones inside their rib cages" (p. 246). To answer this question, it is important to ask what the ribs have to do with the shoulder girdle. Let us start by asking what it is the ribs do.
The functional relationship between the ribs and the shoulder girdle varies. In land animals, ribs give support and maintain the form of the trunk and "afford attachments for axial skeletal muscles," that is, muscles of the trunk (Romer, 1956, p. 56). Some muscles of the shoulder girdle do attach to the ribs. Their function is to hold the "shoulder blade" in place while other muscles move other bones in the forelimb. These forelimb movements are performed mainly by muscles which connect the bones of the forelimb to other bones in the shoulder girdle or to the spinal column.
The ribs are not necessary for the functioning of the shoulder girdle, except that they give the body its form. They are convenient to the shoulder girdle in many modern land animals, but in the fishes and the earliest land animals the shoulder girdle was associated with the dermal bone of the skull.
The shoulder girdle and the ribs form two separate sets of bone and muscle systems. The shoulder girdle is for support and locomotion. The ribs are for form, support, and attachment of trunk musculature. The normal course of reptilian development confirms this separation. The trunk skeleton forms on the "back" section of the embryo, and the shoulder girdle develops on the belly side. Turtles follow this reptilian pattern, too.
Despite the noticeable change in form, the way that the ribs and shoulder girdle function and their operational relationship to each other are essentially unchanged in the turtle. Their relative locations are changed, but this is not significant from a functional standpoint.
We are led to the conclusion that the second creationist objection, concerning shared ancestry, is not supported by the course of development of the turtle embryo or by the mechanics of the trunk muscle-ribs and shoulder girdle functional complexes.
Turtle Phylogeny
The study of turtle embryology demonstrated the process by which a seemingly unusual adult form is produced. Carr's question about the set of conditions which would favor the development of an external shield remains with us, however. What evidence is there for the potential among reptiles to shield their bodies with hard tissues in the skin?
Turtles are covered with a shell of bone. It is the most prominent part of their anatomy to even casual observers. A more careful examination reveals a covering of horn or keratin covering the bony shell. Keratin is a hard substance that forms our finger nails and hair and the claws, horns, and spikes of many other animals. One of the most important of reptile adaptations was the development of this horny layer around the outside of the body. Keratin prevented extreme water loss, and it allowed reptiles to live in more places on the land—much farther from water than the amphibians could travel.
L. B. Halstead reports not only that keratin is a typical covering for reptiles but that some develop small plates of bone deeper in the skin. He goes on to say that the so-called hard keratin of nails, claws, and horn is readily calcified. Adding calcium to keratin makes the tissue harder and stronger but does not produce bone. A body covering of even hard keratin is not likely to be preserved in the fossil record, because its chemical composition and properties are very different from the more frequently preserved hard tissues of bone and teeth.
Whether or not it was calcified to some degree, a hard, external armor made up of horny plates could easily have been the basis of an adaptive shift. Such shields are common among reptiles. The later development of bony armor which could be preserved in the fossil record is also not unusual for reptiles. Halstead leads us to the conclusion that it is chiefly the extent of the development of dermal bone in the skin which distinguishes the turtle from its reptilian relatives. The fact that this dermal bone forms in the skin without a cartilage model makes it precisely the sort of bony shield evolutionary biologists would predict for a reptile committed to enclosing itself in armor. The fact that it forms directly in the skin accounts for its location outside the limb bones.
The reduction of the rib skeleton is also to be expected under these conditions. The shell, once it became complete, would provide form and support for the body. The ribs would not be necessary for this function, and the muscles which move the trunk could attach themselves to the bony shield. In fact, the turtle's ribs do not disappear but become incorporated into the shell. Since the shell is outside the body, the ribs are too.
The studies of embryology show the importance of the shell in the turtle's development and confirms its central role in the turtle's adaptation to its environment (Romer, 1924, 1956; Walker, 1947, 1969). Despite this drastic change of emphasis, Walker (1947) and Zangerl (1969) reiterate that the turtle is a "good" reptile—that is, development and basic structure remain conservative for this class of animals.
A plausible way to develop a turtle from a basic reptilian ancestor has been proposed. It is plausible because it relies on structures and developmental processes which we can observe in living animals and because it is based upon the natural laws which we have observed operating in so many other cases. No new or special mechanism is necessary to explain the result.
Summary and Conclusion
Turtles that are related to other reptiles by a common ancestor should have the following features in common with other reptiles: (1) the form and function of the structures should be developed on the same basic plan; (2) structural modifications should be derived from known anatomical features in the ancestral form; (3) the derivation should be accomplished by means of processes known to exist among the relatives.
Examining these three points for the turtle, we see that all three conditions are satisfied. The function and form of the shoulder girdle of the turtle follow the basic reptilian plan. The dermal bone in the girdle becomes a part of the dermal bone of the lower shell. No new or unique elements appear in the turtle shoulder girdle that distinguishes turtles from primitive reptiles found earlier than the Triassic (195-225 million years ago), according to Romer and Carroll.
The form and function of the turtle ribs are modified by their attachment to the carapace. Some trunk muscles still attach to the ribs, but the function of giving support and shape to the body is yielded to the shell. The ribs fuse with the shell on the back in the same way that the elements of the shoulder girdle fuse with the lower shell.
The processes which might explain the shift toward external armor are present in varying degrees among close relatives. The outermost layer of horn is one of the features that distinguishes all reptiles from their ancestors, the amphibians, and fishes. External armor from horn, calcified keratin, and dermal bone are common among the reptilian relatives proposed for the turtle. The turtle needs no new processes or structures. It uses its existing potential for bodily shields.
Studies of development (ontogeny), evolutionary history and relationships (phylogeny), and functional analysis combine to support a common ancestry of turtles with other reptiles, functional similarity of turtles with other reptiles, and a plausible evolutionary pathway from generalized reptile to a specialized turtle.
Rather than posing a dilemma for the evolutionary biologist, the turtle is a prime example of how a commitment to a new adaptive strategy can have a far-reaching impact upon a whole lineage of animals.
No special creation is needed to explain this accomplishment, however. Only a shift in developmental processes to accommodate a commitment to external armor is needed. There is no fundamental reorganization of form or function which is not associated with the development of the turtle's shell.
