Summary of problems with claim:
The basic problem with this claim, as with the one before, is it relies on a fabricated simplistic assumption, a strawman, that, "[a]ccording to neo-Darwinian theory, the development of non-homologous structures should be regulated by non-homologous genes." (pg. 44)
Full discussion:
Explore Evolution presents this example:
Consider, for instance, the eyes of the squid, the fruit fly, and mouse. The fruit fly has a compound eye, with dozens of separate lenses. The squid and mouse both have single-lens camera eyes, but they develop along very different pathways, and are wired differently from each other. Yet the same gene is involved in the development of all three of these eyes.EE, p. 44
Darwin admitted the evolution of a structure as complex as the eye was difficult, but not impossible, to imagine. Darwin hypothesized that a complex eye could develop through a gradual transition from some type of prototype or simple eye. In fact, anatomists have discovered numerous intermediates between the more primitive prototype and the vertebrate eye. The full range of transitional structures has been observed within living snails (see Salvini-Plawen and Mayr, 1977, "On the evolution of photoreceptors and eye," Evolutionary Biology 10:207-263).
If indeed the vertebrate eye developed from intermediates found in other groups, wouldn t we expect to find some of the same genes involved in the organization of these structures? At the developmental level, according to Carroll, both the mouse Pax6 and the fruit fly ortholog eyeless are involved in regulatory networks that direct eye development. It is therefore not surprising that we find the same gene involved in eye morphogenesis, even when the general morphology of the eye shows variation.
The ancestor of insects and vertebrates would have had light sensitive organs of some sort, and some regulatory gene would have controlled the development of such structures. That gene is a shared, derived trait uniting many groups of animals, including insects and vertebrates. From that ancestral population, one group went on to produce the compound eyes associated with insects, a trait that is a synapomorphy within the arthropods (a group including insects, lobsters and similar species). The vertebrate eye's particular anatomy is a shared, derived characteristic within that group. The consistency of such hierarchies of synapomorphies is what evolutionary biologists use to identify the patterns of common descent within living things.
Responding to a claim similar to that in EE, David Cannatella explained:
Here the faulty logic lies in equating different hierarchical levels, the beginning and ends (genes and eyes) of the developmental cascade. The presence of the Pax-6 gene is probably a synapomorphy of a large group of metazoans, and thus the Pax-6 genes are homologous. But the distribution of the character state "eyes present" on the phylogeny of metazoans requires homoplasy, and the eyes of insects and vertebrates are independently evolved.David Cannatella (1997) "Review of Homology. The Hierarchical Basis of Comparative Biology. by Brian K. Hall and Homoplasy. The Recurrence of Similarity in Evolution. by Michael J. Sanderson; Larry Hufford", Systematic Biology 46(2)366-369.
According to Wagner (2007) the more parsimonious interpretation of the genetic similarity between the vertebrate and insect eyes is that Pax6 is part of the ancestral cell-differentiation pathway for photoreceptors and was then separately incorporated into the identity networks for both types of image forming eyes.
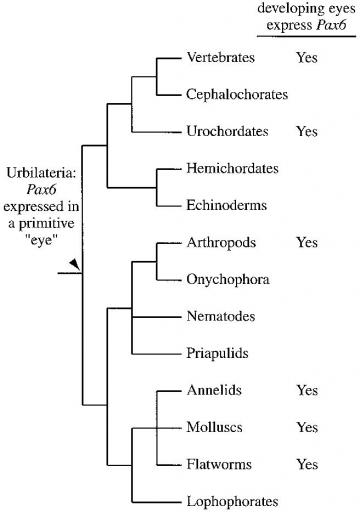 Evolution of eye development: The pattern of species in which Pax-6 is involved in eye evolution indicates that it played a role in the development of light-sensitive structures in the common ancestor of modern bilaterians.
Evolution of eye development: The pattern of species in which Pax-6 is involved in eye evolution indicates that it played a role in the development of light-sensitive structures in the common ancestor of modern bilaterians. Sean B. Carroll, Jennifer K. Grenier, and Scott D. Weatherbee (2001) From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design, Blackwell Publishing:Cambridge, MA, Fig. 3.5 pg. 59
The above observation also allows biologists to form some testable predictions, an important part of any inquiry-based process, disappointingly absent from the supposedly inquiry-based Explore Evolution. We should expect to find Pax6 expression involved in eye morphogenesis in all the descendants of Urbilateria, the common ancestor of insects and vertebrates. The figure at right shows Pax6 that is indeed expressed during eye development in many bilaterian phyla, confirming the evolutionary prediction.
Detailed research on the genetic control of eye development has revealed that the role of pax6 and eyeless is far from the simple story that Explore Evolution presents. A review in 2006 explained that:
Historical views on eye evolution have flip-flopped, alternately favoring one or many origins. Because members of the opsin gene family are needed for phototransduction in all animal eyes, a single origin was first proposed. But subsequent morphological comparisons suggested that eyes evolved 40 or more times independently; this finding is based on, among other things, the distinct ontogenetic origins of eyes in different species. For example, the vertebrate retina arises from neural ectoderm and induces head ectoderm to form the lens, whereas cephalopod retinas result from invaginations of lateral head ectoderm, ultimately producing an eye without a cornea. Multiple origins were also supported by an elegant simulation model. Starting from a patch of light-sensitive epithelium, the simulation, under selection for improved visual acuity, produced a focused camera-type eye in less than 4 x 105 generations. For animals with generation times less than a year, this would be less than a half million years.
>The idea that eyes arose multiple times independently was challenged by the discovery that a single developmental gene, pax6, can initiate eye construction in diverse species. However, subsequent work has shown that pax6 does not act alone and that building an eye requires suites of interacting genes. Discussion about the evolutionary origins of eyes was invigorated by the discovery that homologous genes can trigger construction of paralogous systems for photodetection, just as homologous hox genes do for paralogous body parts across phyla.
For Drosophila photoreceptor arrays, it is now known that seven genes [eyeless (ey), twin of eyeless (toy) (both of which are pax6 homologs), sine oculus (so), eyes absent (eya), dachshund (dac), eye gone (eyg), and optix] collaborate. These genes, in combination with the Notch and receptor tyrosine kinase pathways and other signaling systems, act via a complex regulatory network.
Deletion of any one of the seven genes causes radical reduction or complete loss of the Drosophila eye. Yet in collaboration with certain signaling molecules, any one of them, except sine oculus, can cause ectopic expression of an eye. Like other developmental cascades, a network of genes is required for organogenesis. Six1, Dach, and Eya are important in the formation of the kidney, muscle, and inner ear, as well as eyes, which suggests that this suite of genetically interacting gene products may have been recruited repeatedly during evolution for formation of a variety of structures.
Appearance of photodetection systems probably happened many (possibly hundreds of) times, until selection produced at least the two independent, main types of photoreceptor types known today ciliary and rhabdomeric.Russell D. Fernald (2006) "Casting a Genetic Light on the Evolution of Eyes" Science 313(5795): pp 1914-1918
The pax6 gene family is not the only gene with a role to play in eye development, and the particular combination of genes which produce eyes in modern species was assembled by a process of gene duplication, mutation, recombination, and natural selection. Parts of those developmental pathways are homologous across many species, but other aspects were assembled from preexisting combinations of interacting genes active elsewhere in the body which were drawn together independently, perhaps as many as 40 different times over the evolutionary history of life.
This means that the possession of pax6 is a shared, derived character across the bilaterians, but the particular expression of pax6 in the development of the eye is a shared, derived character within smaller subgroups, a trait which evolved several times, built on the nested hierarchy of evolutionary histories of all those groups.
